Podcast: Play in new window | Download
It’s like an Everlasting Gobstopper, but Willy Wonka is a fratricidal German monk.
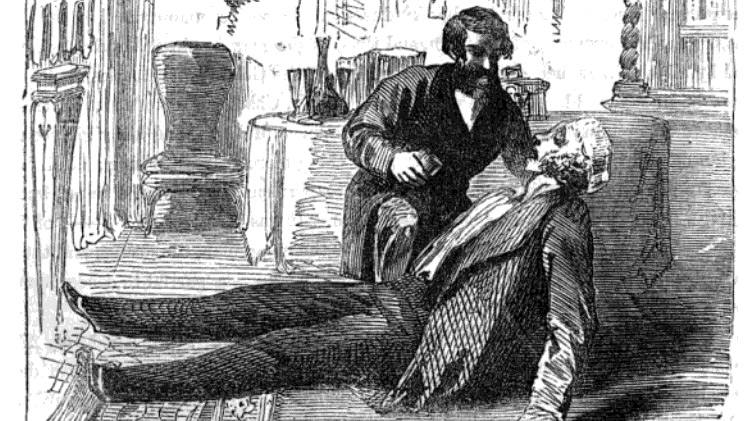
Featured above: A normal Tuesday for William Palmer and friends.
Show Notes
Xenomorph Blood: In addition to the toxicity and the explosions, antimony is also a component of the most acidic substance in all known chemistry: Fluoroantimonic acid.
A substance’s acidity is measured by the pH scale. Pure water is a 7 on this scale, perfectly neutral, and numbers lower than seven are increasingly acidic. Additionally, the pH scale is a logarithmic scale, which means that a pH 5 is ten times stronger than a pH of 6. A pH of 4 is ten times stronger than a pH of 5, and 100 times stronger than a pH of 6. And so on.
Lemon juice and vinegar are acids we can commonly find in our kitchens, and they both have a pH around 2. That’ll sure sting if it squirts in your eye, but won’t cause a lot of damage.
Your stomach acid is hydrochloric acid, with a pH of about 1. This is pretty strong stuff — obviously, since it needs to break down whatever food you toss down there. It would seriously damage your insides, if they weren’t coated with a healthy barrier of phlegm.
Sulfuric acid is widely used in industry, and it blows all those away with a pH of -12. We’re talking hundreds of billions of times stronger than stomach acid. It would cause severe, potentially fatal chemical burns to anyone unlucky enough to spill some on themselves.
Anything stronger than this sulfuric acid called a superacid — you can imagine why.
With a pH of -28, fluoroantimonic acid is ten million billion times stronger than pure sulfuric acid.
Obviously these numbers are too huge to make any sense to our primitive ape brains. There’s not even any video I can show you of this stuff — that’s how dangerous it is. It won’t just eat through your skin and bones, but also through any container you store it in. Any container, that is, except for one lined with Teflon.

Overconfidentii Vulgaris: In that episode on sodium, we referred to our devout friend as Basilius Valentinus, whereas here we call him Basil Valentine. Either is fine. He wrote in Latin, so his name would have literally read as Basilius Valentinus, but many translators also de-Latinize his name. Both are accepted as referring to the same person.
Speaking Of Latin: These are the only elements that do not end in -ine, -on, -um, or gen: phosphorus, sulfur, manganese, cobalt, nickel, copper, zinc, arsenic, silver, tin, antimony, tungsten, gold, mercury, lead, and bismuth. As you can tell, most of them acquired their names before Berzelius got his Latin-loving hands all over the periodic table.
Oh hey! I wanted to include that “extremely explicit woodcutting” of the guy with fluids pouring forth from all orifices. This seems as good a spot as any!
By the way, this woodcutting happens to be ripped straight from the pages of Antimony, Gold, And Jupiter’s Wolf.
Imagine What Christmases Were Like: Palmer and his main squeeze appear to have had a real courtship. They wrote letters and everything, but they don’t really speak any better to Palmer’s character. Try to keep your heart from racing while reading such a steamy epistle as this:
My Dear Little Annie,
It was not the rain that prevented me from joining you at Stafford, as you wished. I sprained my foot, and it was so painful that I could not keep it on the ground. I slid off the pathway as I was turning the corner from the ‘yard,’ past Bonney’s. Now you know the reason, I am sure you will forgive me.”
Lest you mourn for Dear Little Annie’s mother, Mrs. Thornton, you should know that you’d apparently be the only one. She had a reputation worth recording like this:
This much is certain, that the poor old Colonel was harassed out of his life by Mrs. Thornton, who was not only an habitual drunkard, and a notoriously bad woman, but was in the habit of chasing him round the room with a knife, swearing she would kill him. So frequent, indeed, were these outbursts, that when at last he was found dead, if it had not been for the Colonel’s statements made a day before he committed suicide, she would undoubtedly have been suspected.”
I Thought I Had Some Niche Hobbies: You learn some weird things while researching the building blocks of the universe. While looking up some details on the use of antimony to harden up bullets, I came across a discussion board that appears to be devoted to the craft of making one’s own bullets. Maybe it’s more complicated than that, but I can’t really say, since I didn’t stay for very long.
Episode Script
Hello listener, just a quick note that today’s episode involves a rather surprising amount of explicit talk about some deaths and bodily fluids of every variety. I promise it’s all relevant, but if that’s not quite your cup of tea, I don’t blame you for sitting this one out.
The elements of Group 15 stand out on the periodic table for their significant and differing effects in the chemistry of life. The thick, warm atmosphere blanketing the Earth is 78% nitrogen, and the element is an indispensable ingredient in the chemistry of life.
Phosphorus, the next element in the group, is every bit as necessary for life — but it can also poison a person if not treated with respect, and it’s been exploited as an especially cruel weapon of war.
Arsenic is more candid about its ability to kill, being one of history’s most notorious implements of murder. It is possible to befriend this element, but the benefits of such an effort are questionable at best.
Humanity’s history with antimony goes back thousands of years, but the perks of this relationship are even more dubious than the last. Yet despite every red flag raised by today’s element, we seem to live in hope that it might love us back.
Actually, that seems to be the thread tying together all of our stories about antimony: Some things just aren’t true, no matter how badly we wish they were.
You’re listening to The Episodic Table Of Elements, and I’m T. R. Appleton. Each episode, we take a look at the fascinating true stories behind one element on the periodic table.
Today, we’re choking down antimony.
Right off the bat, antimony sounds different from the majority of the elements. It doesn’t end in -ine, -on, -um, or -gen, and in fact, it’s a strong contender for the title of “Element with the Most Fascinating Etymology.”
For one thing, its name’s origins were shrouded in mystery for a long time, which led to some intriguing but ultimately incorrect theories. Let’s knock those out of the way first.
One hypothesis was that element 51’s name comes from the Greek roots “anti-” and “-monos,” translating as “against solitude.” The rationale here is that antimony is practically never found in its elemental form, but rather, it combines with many other elements to produce a wide variety of compounds. That sounds plausible, at least at first blush… but that’s how most of the elements behave. It’s actually far more notable if you stumble across one that does naturally occur in its pure form.
Another thought is that the name comes from a different Greek root, “anthos,” which means “flower.”1 Ores of antimony can have an eye-catching appearance similar to a blooming flower, so again, this feels possible, but there isn’t much evidence beyond that.
Surely the most colorful tale comes to us from Basil Valentine, a 15th-century monk from Germany. If the name sounds a little familiar, that’s because we studied one of his texts in the Sodium episode. He wrote about the eagle and the dragon and the mineral bath for the king, which all turned out to be less absurd than it sounds.
Regarding antimony, he threw in a slew of the stuff with the slop for the swine, and observed that — after the pigs stopped violently vomiting — they tended to really plump up over the next few days.2
Valentine thought, as anyone would, that his fellow monks could do with some plumping up themselves. He didn’t want to deal with all the hassle of convincing them why that was a good idea, so he simply surreptitiously supplemented their suppers.3
The results of this experiment were calamitous. For now, let’s just say that Valentine was not suddenly surrounded by portly monks. He dared not confess his role in the misadventure — rather out of character for a clergyman, but oh well — so with a heart full of silent anguish, he dubbed the substance “anti-moine,” loosely meaning “monksbane” or “anti-monk.”4
This most outrageous theory is also the easiest to disprove, mostly because people had been calling the material “antimony” for several centuries before the purported incident.
This is pretty typical of Brother Basil’s behavior, though. Let’s just earmark this tale for now — we’ll get back to Valentine in a bit.
The real story of antimony’s name, and its chemical symbol, extends much further back in time.
Makeup is just about as old as dirt. In fact, the earliest cosmetic products were little more than special dirt. Coal, galena, malachite, and other minerals would be applied directly to the skin to impart a desired color.
It was an especially common practice in ancient Egypt. Men and women, young and old, would all use cosmetics, especially eyeliner. Their word for eyeliner was “kuhl,” and most of the time, it was made out of a substance called “sdm.” Today, we recognize that substance as powdered antimony. This is the stuff that provided the dark outline around Egyptians’ eyes that can be seen in some ancient artwork.
It was popular stuff, so it stuck around for a long time — and traveled far. Pliny the Elder and Dioscorides both reference sdm, in Naturalis Historia and Materia Medica respectively, as “stimmi” or “stibi.”
As with so many things, the Romans took the word from the Greeks wholesale, just adding a familiar suffix to the end to make “stibium.”5
You might remember that celebrity Swedish scientist Jons Jakob Berzelius used the Latin names of elements to construct their chemical symbols, even when they didn’t match up with the element’s common name. That’s why antimony’s symbol is S b.
“Stibium” traveled around the Mediterranean, becoming “stimmida,” “uthmud,” “othmod,” and in Arabic, eventually, “ithmid.”6
A common definite article in Arabic is “al.” It’s the same as the word “the.” So if you had wanted to say “the antimony” in Arabic at the time, you’d say “al-ithmid.”7
The Arabian world was hugely influential for several centuries in the medieval period, so a lot of loanwords found their way into European languages. When “ithmid” made its way back into European languages, it brought the “al” with it.
This is actually the case for a lot of words in Western languages, including “algebra,” “alkali,” “alembic”… and “alchemy.”
But if the alchemists loved Arabic, they were fanatical about Latin. So as “al-ithmid” traveled throughout the continent, they just couldn’t resist adding a little “-ium” to the end there. There’s something pretty funny about that — as Peter van der Krogt says on his Elementymology website: “Thus Antimonium is the Latinized form of an Arabization of the Latin Stibium!”
There is one other linguistic curiosity here, just as a brief aside. If you remember the Egyptians, “sdm” was the powdered antimony they used as an ingredient for eyeliner, but the eyeliner itself was called “kuhl.” Well, that word made its way into Arabic, too, as “al-kuhl.” After a while, it didn’t just mean powdered antimony, but any kind of powder — and later, it meant any kind of powerful extract of a substance.
In the sixteenth century, our old pal “Dose Makes The Poison” Paracelsus was working with a distilled extract of wine, and he called it “alcool vini.” It wasn’t long after that Europeans began referring to intoxicating spirits as “alcohol.”8
Anyway, the alchemists got a lot of use out of antimony, because it can appear vastly different under differing circumstances.
In its most stable form, it sure looks like it’s a metal: shiny, lustrous, and grey. But it’s a poor conductor of heat and electricity. When subject to careful heating and cooling, its appearance changes. Antimony can appear black or yellow, like arsenic or sulfur, and lose any metallic properties it appeared to have.
This dual nature is why antimony is now classified as a metalloid. For the alchemists, though, they saw antimony as something that surely must be related to gold somehow. It could have the yellow color of gold, or its metallic lustre, but never both at the same time.
Basil Valentine might have been the number one fan of element 51. Stiff competition on that front, but he really went above and beyond by publishing a text called, “The Triumphal Chariot Of Antimony.” The front matter displayed a parade of antimony, mercury, and other substances represented as mythological figures in grand splendor.
Valentine’s works also had an incredible origin story worthy of its ostentatious title and artwork: Supposedly, they had been secreted away inside a stone pillar in a German abbey. The books were only revealed when a bolt of lightning split the pillar in twain, surely guided by divine providence.9
Johann Tholde was the lucky witness of this miracle, and dutifully translated, edited, and published Valentine’s works. That’s what Tholde claimed, anyway, but all evidence makes it very clear that the works were written by Tholde himself, and he invented Valentine as a pseudonym, either to give the works a little promotional juice, or to absolve himself from any culpability for such outrageous ideas.1011
What sorts of ideas? For one, “The Triumphal Chariot Of Antimony” promoted antimony as a cure for numerous ailments, practically elevating it to the level of panacea.
He wasn’t alone in proposing this. Antimony was widely used as a medicine for centuries, usually because of its potency as a purgative or laxative. One extremely explicit woodcutting from 1673 makes this very clear, illustrating a man with voluminous amounts of fluid erupting from both his ends.
For a while, this was seen as a great remedy for hangovers. While enjoying a raucous night of revelry, one might pour some wine into a goblet of pure antimony. It would steep overnight, suffusing the drink with the element. The next morning, to soothe their aching head, they would throw back this one last libation and wait for these emetic effects to quickly take hold.
Of course, not everyone can afford a fine work of craftsmanship solely dedicated to easing the ill effects of an evening’s entertainment. Luckily for the frugal dipsomaniac, antimony provided perhaps the single most economic remedy in all of medical history: The perpetual pill.
In 1907, Dr. William J. Robinson published The Medico-Pharmaceutical Critic & Guide, which carried the following impressively lengthy subtitle:
A Journal of Individuality. No policy except the policy of Truth, Honesty and Fairness. Every Reader has an equal show with the Editor to express his Opinion. No padding. No dead Matter. Every line interesting. All Frauds and Humbugs fearlessly Exposed.”12
Clearly he had quite a colorful way of writing, in addition to his medical savvy. He described this antimony pill far better than I could hope to, so I shall simply quote him here at length:
…we believe that the everlasting cathartic pill beats everything in the line of economy. This pill was a little bullet composed of metallic antimony which had … the property of purging as often as it was swallowed. … The bullet was passed out, recovered from the feces and used over and over again. This, as Dr. J. A. Paris says, was economy in right earnest, for a single pill would serve a whole family during their lives and might be transmitted as an heirloom to their posterity. We have heard of a lady, says the Doctor, who, having swallowed one of these pills became seriously alarmed at its not passing. “Madam,” said her physician, “fear not. It has already passed thru a hundred patients without any difficulty.”
We do not think that the everlasting pill would be popular at the present time.”13
Indeed, more century later, this still sounds like a fair assessment.
Even if the recovery process weren’t enough to dissuade the modern patient, there remains the fact that antimony is poison.
That’s the entire reason it’s so effective at its job. The body knows it’s dealing with a terrible tenant, and is trying to evict it by whichever door is closest.14
Clearly the fad had passed by the 20th century, but antimony was a popular prescription pretty much right up until that moment. For a time in the 16th and 17th centuries, though, this was quite controversial. Paracelsus and his adherents adamantly argued that antimony was a fine medicine, because they had tried it, and seen from experimentation that it yielded the intended results.
Philosophically opposed were the Galenists, practitioners of the classical school of medicine originally taught by the ancient Greek physician Galen of Pergamon. Most notably, they believed that the body is governed by the four humours — blood, phlegm, black bile, and yellow bile — and illness was caused by an imbalance of these liquids. Antimony did nothing to affect the humours, they said, and so it could be nothing more than poison.15
This conflict was dubbed The Antimony War. Not a drop of blood was shed, though it involved a fair amount of other bodily fluids.16
What was at stake? Nothing less than a world-changing paradigm shift: Empirical science versus the status quo. It was hard-fought, and the Galenists had quite a bit of institutional support (as the status quo often does), but the humours were just about put to rest by the turn of the 18th century.17
Never mind that antimony is, again, a highly toxic substance that the body neither needs nor wants. The empiricists were preaching the right method, but a very wrong conclusion.
Paracelsus, full name Philip Theophrastus Aureolus Bombastus von Hohenheim, would likely have brushed aside this concern. As you know, he believed that poison is a relative term.18
So that is why antimony continued to be prescribed for several hundred years after the Antimony War.
Not everyone was as skilled as Paracelsus at measuring a proper dose, though, and antimony’s history is not without its share of tragedy. Often, this tragedy was abrupt and appalling, because strangely, a dose that’s fine for one person can be fatal for another, and a patient who appears healthy can take a most unexpected turn for the worse.
For instance, in 1854, a young girl took a dose of antimony and vomited for several hours thereafter. (That was the intended effect.) The next day, she seemed right as rain, but she started vomiting again that afternoon, and before nightfall she had died.
But of those who have been claimed by accidental overdoses of antimony, one notable name stands out far above all others.
…Mmmmmmaybe.
The classical composer Wolfgang Amadeus Mozart suffered a remarkably puzzling death. There’s disagreement on even the most basic facts of the circumstances. All that’s certain is that when he was thirty-five years old, he became so ill that he couldn’t leave his bed. After two weeks of swelling, spewing, soreness, and suffering, he succumbed.19
A popular and salacious belief, even today, is that he might have been intentionally poisoned by a jealous rival. If you’ve listened to the episode on arsenic, you might remember I said that historians agree that Mozart was almost certainly not poisoned.
For the record, historians agree that Mozart was almost certainly not poisoned.”
I am not backpedaling here, because, for the record, historians agree that Mozart was almost certainly not poisoned.
At least, not intentionally.
It’s more plausible that Mozart might have accidentally poisoned himself. He was something of a hypochondriac, and regularly swallowed medicines containing today’s element. When he became feverish in his last days, doctors administered medicines containing even more antimony.
He had symptoms to match: the vomiting, of course, but also swelling all over his body, terrible body odor, and a rash that is sometimes associated with antimony poisoning.
The problem is, the many symptoms he had are all vague enough to elude a definitive diagnosis. Equally persuasive arguments have been made that he died of syphilis, trichinosis, streptococcus, vasculitis, glo-MER-u-lo-ne-phri-tis, and other, more obscure diseases.20 With so many possibilities, it’s effectively impossible to conclude the real cause of death — especially since the patient has underground for the last two centuries.21
Mozart’s is one of history’s most infamous unsolved deaths, and will surely continue to intrigue amateur pathologists for all time.
Sometimes, though, the method by which a person has died is not as important as the simple fact that a person has died. Because for all of the accidental death associated with the element, antimony has certainly been used as an device of more deliberate deaths, as well. Among the worst perpetrators of such crimes was a man named William Palmer.22
Palmer was an English doctor in the mid-nineteenth century who seemed to be something of a bad luck charm. In 1846, he challenged a man to a drinking contest; that man died in his bed later that night. No one ever proved anything, or even suspected anything at the time — but it was noted that Palmer fancied his competitor’s wife.
He didn’t stick around, though. For unspecified reasons, Palmer returned to his hometown of Rugeley, where he practiced as a physician. There he met Ann Thornton, and the two were soon married.
Who knows what motivates one in matters of the heart? We don’t know what the young Ms. Thornton saw in Palmer, but it appears that Palmer looked a little bit past his betrothed and saw her mother, who had recently inherited a small fortune of eight thousand pounds.23
She came to live with the newlyweds, but her tenure was brief. Only two weeks later, Mrs. Thornton had tragically perished. The doctor recorded the official cause as “apoplexy,” a word sometimes used to indicate a stroke.
What terrible luck!
Lending money to Palmer was the quickest way to ensure an imminent departure, especially because he had a tendency to lose it all, and swiftly, too. Technically he was still a doctor, but practically, he was spending most of his time down at the racetrack.
To wit: He befriended a fellow gambler named Leonard Bladen, who generously lent Palmer six hundred pounds. Curiously, Bladen died shortly thereafter. At Palmer’s house. His recent winnings were nowhere to be found, nor were the books that recorded his loans.
An “abscess in the pelvis” was the root cause of this untimely death, according to the doctor.
Over a period of three years, Ann and William had four children… and also lost four children. To “convulsions,” if you believe the death certificates. Palmer was reputed to have even more children out of wedlock, all of whom promptly met the same fate.
By 1854, Palmer was running out of clears throat benefactors, and his remaining family members possessed no great wealth.
But Palmer was not out of ideas. He decided to take out a generous life insurance policy on his wife. This proved to be the prudent thing to do when, sadly, she passed only a couple months later. Palmer received thirteen thousand pounds in compensation.
A very similar situation unfolded when his brother died the following year, but the insurance company wasn’t so eager to pay up. Investigators soon discovered that Palmer had taken out another insurance policy on a former employee — without that employee’s consent, or even knowledge of the arrangement. The insurance company refused to pay out the policy.
A couple months later, Palmer was cozying up to John Cook, another friend from the racetrack who was on a lucky streak. Cook noticed that anything he drank tended to burn his throat whenever Palmer was around. A week later, Cook died, suffocating in his own bed. The official cause of death was ruled “apoplexy.” Haven’t we heard that one before?
Cook’s stepfather saw that something strange was going on, and began an investigation.
A truly bizarre autopsy took place, conducted by a medical student and his drunk assistant, with Palmer comically bumping into the practitioners at the most inopportune times and carrying off containers of vital fluids for “safekeeping.” Palmer later wrote a polite note to the coroner requesting that he decree that Cook died by natural causes, and very thoughtfully included a ten-pound note.
The investigation formally found that John Cook had “died of poison willfully administered to him by William Palmer.”
Palmer was prosecuted for murder by strychnine, though he had a record of purchasing opium and other toxic substances. When medical examiners exhumed his late wife’s body, antimony was found in all of her organs. It seems that his medical knowledge didn’t go entirely to waste.
A swift trial condemned Palmer to hang by the neck until dead. When asked to admit the crime for which he was just found guilty, Palmer said, rather cryptically, “Cook did not die from strychnine.” The official’s response, “This is no time for quibbling. Did you, or did you not, kill Cook?” Said Palmer, “The Lord Chief Justice summed up for poisoning by strychnine.”
When he was handed over to George “Throttler” Smith at the Old Bailey, Palmer motioned toward the gallows’ trap door and said, “Are you sure it’s safe?”
Of the thirty thousand people who witnessed Palmer’s end, the history books only mention one who was distraught. His mother wept and cried out, “They have hanged my Saintly Billy!”24
I trust that if I lend you any advice on how to procure today’s element, it’s strictly for inclusion as a display piece in your chemical museum, and you’ll take sufficient care to protect yourself and those around you. You’ll need to go a little out of your way to find it, and there is yet one more way antimony could kill you, so do listen through till the end.
Bullets commonly contain a small amount of antimony, although that’s not what I was getting at. Lead is still the metal of choice to lend bullets the kind of mass that will really deliver a wallop, but it’s just a little too soft as the pure thing. A few percent antimony will harden that right up, and that’s been part of the process for centuries.25
That hardening property has historically been the second-most popular use for antimony, right after its ability to bring your insides out. Church bells and type blocks for Gutenberg’s first printing presses both used antimony as a hardening agent, but today you’re likely to see that application in lead-acid car batteries. That’s actually where most industrial antimony ends up as we round out the year 2019. Electric cars use a different kind of battery, so if you’re listening to this in the future, hopefully this is no longer true.26
You might not expect a coin to be among the options for an element that is not, strictly speaking, a metal. But it is!
Guizhou is a rural province in China, and in 1931, the people who lived there had a hard time getting their hands on currency. The solution, they decided, was to mint some of their own. That’s a pretty good idea, except they didn’t really have any supplies of what would traditionally be called coinage metals.
What they did have, as you probably guessed, was antimony. Alloying that with lead, they struck around half a million coins. They weren’t well received, though. On top of being made of two quite toxic elements, the coins were very brittle and wore down easily.
After that initial run, Guizhou never made any more antimony coins, and nobody really used them. Nowadays, the paltry little coin that no one wanted can rarely be found, and then, only for thousands of dollars.27
One of the main sources for antimony, in Guizhou and elsewhere, is the element’s sulfide, called stibnite. It’s a beautiful, shiny dark mineral that can look like a starburst in crystal form.28
Elemental antimony looks kind of similar, most of the time, but less striking. Basically just a shiny grey lump. Even the discerning collector might prefer one of its other allotropes, just for a little variety.29 Yellow and black antimony are two purely elemental samples, but you should probably avoid antimony that looks like glass.
That allotrope, when subjected to the slightest provocation — heat, or impact, or even just a slight scratch — will violently explode.30 31 32
So it’s probably not worth keeping any around. It’s just not worth the risk of leaving behind nothing but a smoldering crater… and yet one more mystery attributable to antimony.
Thanks for listening to The Episodic Table of Elements. Music is by Kai Engel. To learn about a completely different way antimony can be hazardous to your health, visit episodic table dot com slash S b.
Next time, we’ll sniff out tellurium.
Until then, this is T. R. Appleton, reminding you that it sure is wonderful to be alive now, when the practice of medicine is slightly less horrifying than at any prior point in human history.
Sources
- Journal Of Sustainable Metallurgy, Antimony Recovery From End-Of-Life Products And Industrial Process Residues: A Critical Review. David Dupont et. al, March 2016.
- The Devil’s Doctor: Paracelsus And The World Of Renaissance Magic And Science, this page. Philip Ball, 2006.
- A Technological Dictionary: Explaining The Terms Of The Arts, Sciences, Literature, Professions And Trades, p. 82. W. M. Buchanan, 1842.
- The Useful Metals And Their Alloys, p. 604. John Scoffern, 1857.
- Elementymology & Elements Multidict, Stibium/Antimony. Peter van der Kroogt.
- Educational Notes And Queries: A Medium Of Intercommunication For Teachers, Volumes 5-6, p. 120. Edited by William Downs Henkle, 1879.
- Folk-Etymology: A Dictionary Of Verbal Corruptions Or Words Perverted In Form Or Meaning, By False Derivation Or Mistaken Analogy, p. 460. Rev. A. Smythe Palmer, 1882.
- The Devil’s Doctor: Paracelsus And The World Of Renaissance Magic And Science, this page. Phillip Ball, 2006.
- Science, Note On The Age Of Basil Valentine. C. S. Pierce, August 12, 1898.
- Alchemy And Finnegan’s Wake, p. 97. Barbara DiBernard, 1980.
- The Historical Background Of Chemistry, p.98. Henry Marshall Leicester, 1971.
- The Medico-Pharmaceutical Critic & Guide, Dr. William J. Robinson. 1907.
- ibid., p. 55.
- McGill Office For Science And Society, Antimony: A Metallic Cleanse Of The Middle Ages. Dr. Joe Schwarez, February 15, 2017.
- The Story Of Chemistry, this page. Anne Rooney, 2017.
- U.S. National Library Of Medicine, Antimony, Elemental.
- Chemistry World, Antimony. Philip Ball, May 18, 2010.
- Encyclopedia Britannica, Paracelsus. John C. Hargrave, last updated December 6, 2019.
- The Elements Of Murder, p. 220-225. John Emsley, 2006.
- The New York Times, What Really Killed Mozart? Maybe Strep. Nicholas Bakalar, August 17, 2009.
- PBS NewsHour, A Symphony Of Second Opinions On Mozart’s Final Illness. Dr. Howard Markel, December 5, 2016.
- The Elements Of Murder, p. 225-229. John Emsley, 2006.
- Illustrated Life And Career Of William Palmer Of Rugeley, p.32-35. Published by Ward & Lock, 1856.
- Dark Secrets Of The Black Museum, 1835-1985: More Dark Secrets From The Most Notorious Crimes In England, this page. Gordon Honeycombe, 2014.
- The Balance, History: Antimony Metal. Terence Bell, April 26, 2018.
- Umicore, Antimony. I wouldn’t trust this or any industrial source for etymological research, but in terms of modern-day industrial applications, seems legit.
- Slate, Antimony: It Might Have Killed Mozart. Sam Kean, July 7, 2010.
- Antimony: Its History, Chemistry, Mineralogy, Geology, Metallurgy, Uses, Preparations, Analysis, Production, And Valuation; With Complete Bibliographies. Chung Yu Wang,1919.
- Chemistry Of Arsenic, Antimony, And Bismuth, p. 48-52. N.C. Norman, 1997.
- The Chemistry Of Antimony, p. 11. Chung Yu Wang, 1909.
- Chemistry Of Arsenic, Antimony, And Bismuth, p. 50. N. C. Norman, 1997.
- Proceedings Of The Royal Society Of London, Studies On Explosive Antimony. C. C. Coffin, October 15, 1935.

Did you watch any of the Periodic Table of videos, T.R Appleton? These videos are very good information about the information and chemistry of the elements
Yes! I’m a huge fan of those, they’re a big inspiration!
Technically, iron doesn’t end in a -on ending in non-English languages.
BTW, have you listened to the Chemistry In it’s Element podcast? Good source of element information.
Have you answered my questions yet?
You’re hadn’t answer them since a fortnight ago
Hi! Sorry to take so long to reply, I’ve been so busy lately. I have listened to Chemistry In Its Element! It almost always provides a good jumping-off point for my research.
Any Pnictogens left remaining in the periodic table?
Definitely! Bismuth, for sure, and moscovium might behave like the other pnictogens, too.
You seem very interested in the named groups! Any reason in particular?
You meant the main groups on the table; the p and the s- blocks? Btw any chalcogens left on this table?
Anyway I like the main groups but particularly the p-block ones.
Your story about the name seems to end with “ium” being added to the end. What’s the rest of the story: how it got from al-ithmid-ium to antimony? Thanks for the super podcast. I loved it.