Podcast: Play in new window | Download
Truly strange things start to happen when you breathe this rarefied air.
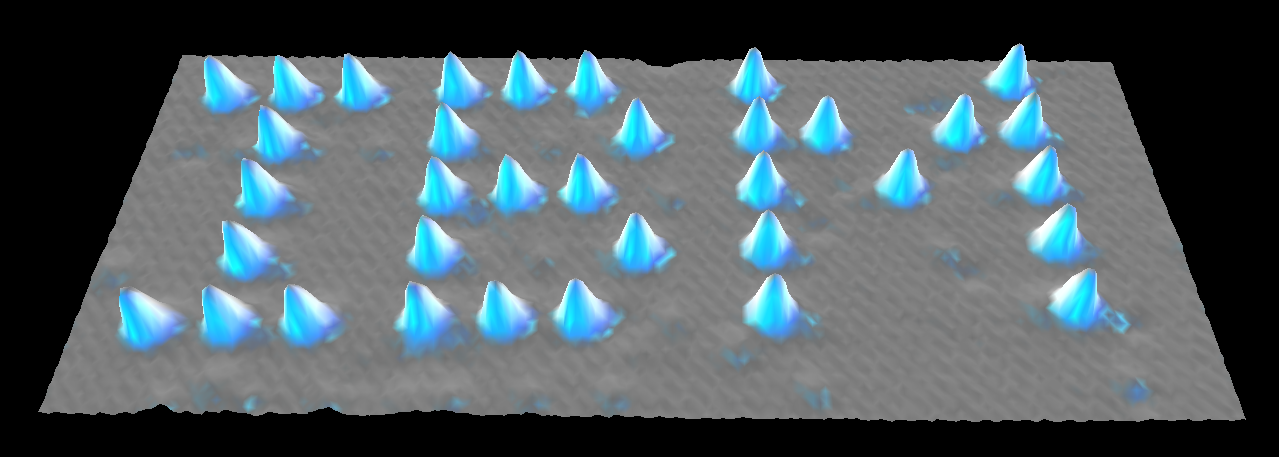
Featured above: The world’s smallest advertisement.
Show Notes
The Lazy Academic: One of the books I consulted for this episode had a really great name: Some Nineteenth Century British Scientists. Nothing comprehensive, no reason for the theme… just, y’know, some nineteenth century British scientists.
Microscope Under A Microscope: Here’s a pretty good animation of how the STM works, courtesy Jubobroff on Wikipedia:
Smallest Screen: In 2013, IBM upped their microphotography game by creating a stop-motion animated picture:
I’m Sure This Was Obvious: In mentioning that platinum hexafluoride can oxidize oxygen, I should mention that I’m specifically talking about diatomic oxygen, O2. It’s remarkable because the double bond between those two atoms is incredibly strong. It takes something really strong to break those two up. In its monatomic form, oxygen has six valence electrons, and readily swaps them with other atoms.
 He Had A Particular Set Of Skills: Neil Bartlett’s experiment was ingenious enough on its own, but he also had to blow all the glassware for his experiment himself. (See left.) He didn’t even get any help from his graduate students — they were terrible glassblowers.
He Had A Particular Set Of Skills: Neil Bartlett’s experiment was ingenious enough on its own, but he also had to blow all the glassware for his experiment himself. (See left.) He didn’t even get any help from his graduate students — they were terrible glassblowers.
Apparently it’s something of a dying art. We need more glassblowers!
They Can’t All Be Winners: As we’ve noted before, not all scientists are great poets. Immortalization by bawdy rhymes might be a great honor, but preferably the author respects the meter a little more than the person who wrote this limerick. It took some creative recitation to make this one sound acceptable!
Original Research: There’s a fairly sizable body of research that indicates redheads require more anesthesia to receive an adequate painkilling effect (although not everyone agrees). Xenon works differently from pretty much every other anesthetic, though, so I can’t help but wonder if it would be a better choice for red-haired patients in the operating room. As far as I know, there’s no research on this, yet!
Renault Missed Out: Vichy Water is a naturally carbonated mineral water that comes, naturally, from Vichy, France. Curiously, it also happens to contain small amounts of argon, krypton, and xenon. They bubble up from elements undergoing radioactive decay deep underground. It’s a nice little bonus to go along with your fizzy water!
Episode Script
Scientific innovations are not always met with approval and excitement, believe it or not. For instance, after the 2014 Winter Olympics in Sochi, officials were quite displeased to learn that Russian athletes had found a new way to artificially boost certain hormone levels — and that might’ve helped them take home more medals than anyone else that year.
A Russian coach insisted that there had been no wrongdoing. “We use what is not illegal, is not destructive, and does not have side effects.”1
Technically true! The Russians had reaped those hormonal benefits by inhaling a 50-50 mixture of oxygen and xenon. The practice doesn’t really have any side effects; it’s not destructive; it’s not against any rules or bylaws. Or at least, it wasn’t, at the time.
That didn’t last long, of course. In a matter of weeks, the World Anti-Doping Agency banned Olympic athletes from breathing xenon gas, as well as its lighter sibling, argon.2 3
This doping scandal is not the only crime on xenon’s rap sheet. It turns out that xenon has a habit of breaking the rules — even the very rule that defines what it means to be a noble gas.
You’re listening to The Episodic Table Of Elements, and I’m T. R. Appleton. Each episode, we take a look at the fascinating true stories behind one element on the periodic table.
Today, we’ll make the acquaintance of xenon.
Few people filled out their periodic bingo card better than William Ramsay. By July 1898, he had discovered neon, “the new one,” argon, “the lazy one,” and krypton, “the hidden one.” As a side project, he also helped some Swedish chemists discover terrestrial helium.4 Now he was about to make five in a row with the discovery of xenon, “the stranger.”
Ramsay and his assistant, Morris Travers, had pulled noble gases from the atmosphere by distilling the air, then distilling the product of that distillation, then distilling the product of that distilled product. They couldn’t do it again, though — their equipment simply wasn’t good enough to apply any greater pressure to the remaining gas.5
Thankfully, they had a benefactor in British German industrialist Ludwig Mond, who gifted them a state-of-the-art air compressor in support of the cause.6 With this, they were able to go beyond anything they had done before — past neon, further than argon, beyond krypton. Finally, they condensed the air down to a tiny fraction of a tiny fraction of a tiny fraction — and only then did they find xenon.7
Each step was a shift in orders of magnitude. Kind of like in the movie Ant-Man, in which Scott Lang uses a special suit to shrink to the size of an ant, then shrinks again to the size of bacteria, and so on, until he’s smaller than individual atoms.
…
While he was down on that level, Scott Lang could have visited a real-life microscopic masterpiece: IBM in Atoms.
In 1989, IBM employees Dr. Donald Eigler and Dr. Erhard Schweizer were doing research with a state-of-the-art scanning tunneling microscope. An STM doesn’t work like the microscopes found in a high school biology lab. It’s looking at things that are simply too small for a traditional lens to see.
Instead, the STM acts kind of like a record player. It operates using a needle with the sharpest tip possible — only one or two atoms wide. This tip is then dragged over a surface in straight parallel lines and measures the height of the scanned area with accuracy down to a tenth of a nanometer. You can’t really say the STM takes photographs, but it does create an elevation map that shows individual atoms.8 9
That is a phenomenal scientific triumph, but Eigler and Schweizer were more interested in something else the machine could do: it could bring the tip a little too close to an atom, pluck it off the surface, then drop it elsewhere: Fine manipulation of individual atoms. It doesn’t work with every element, but xenon atoms are stable and rather large in radius, making them easy to manipulate in this fashion.10
The two scientists started nudging xenon atoms around on a perfectly smooth and incredibly cold nickel surface. After twenty-two tedious hours, they had arranged thirty-five atoms of xenon in a way that spelled out their employer’s initials: I B M.11
It was a breakthrough moment for nanotechnology — the beginning of practical work in a field that had been purely hypothetical for thirty years.12 13 14
Rarely are those who work in the sciences hailed for their artistic achievements, but this was an accomplishment in both regards. Eigler and Schweizer had used the world’s finest brush to compose history’s smallest painting: A logo in xenon.
Xenon can pull off all sorts of neat tricks under extreme conditions. In 1978, a team at Cornell University crushed xenon under the pressure of hundreds of thousands of atmospheres, and it suddenly became something new, something that conducted electricity as well as steel: A form of xenon that was a metal.1516
That’s really weird, but it’s also in keeping with trends we’ve seen on the periodic table. As you travel down the periodic table, the elements get larger, and electrons have to orbit the nucleus at a farther distance once the inner orbitals are filled. Being farther away, those electrons are less tightly bound to the nucleus, and are easier to lose.17
You can think of it like the parking lot at a basketball game. The first parking spaces to get taken are going to be those closest to the venue, and they’ll get taken quickly. Latecomers will have to settle for a parking spot that might require a bit of a hike to the arena.
When the game is over, everyone will be trying to leave at the same time. The cars parked nearest the venue are going to spend a lot of time stopped in heavily congested traffic, but the cars parked farther away will have an easier time making it back onto the road. Xenon’s outermost electrons are kind of like those cars parked at the edge of the lot.18
The ability to easily lose electrons is what allows metals to exhibit their characteristic behavior — malleability, ductility, conductivity, and lustrousness. So xenon just needed a little nudge — on the order of 320 kilobars of pressure — to push it over the line and become metallic itself.
Truly bizarre. And yet, scientists have goaded xenon into even more outrageous behavior, the kind that’s most unbecoming for a “noble” gas. They’ve managed to convince xenon to bond with other atoms.
You probably remember that the one defining feature of Group 18 elements is that they don’t interact with other elements. Their valence shells are filled to capacity. The neither wish to gain nor lose any electrons. Other atoms simply have nothing to offer the noble gases.
But xenon can be intimidated into action. Once again, that’s because of its distantly orbiting electrons, which are a little more loosely bound to the nucleus than the electrons closer to the center.
The amount of energy it takes to knock a valence electron away from its parent atom is called “ionization energy.” Xenon’s ionization energy is still quite high — it’s still a noble gas, after all — but in 1962, Dr. Neil Bartlett showed that ionizing xenon is just this side of possible.19
Bartlett had recently been doing some work with platinum hexafluoride, a chemical that’s such a strong oxidizer that it can oxidize oxygen. As a reminder, oxidizing agents are chemicals that can strip electrons away from other atoms.
After a little study, Bartlett wondered if platinum hexafluoride could be such a strong oxidizer that it could do the impossible: Combine with an “inert” gas.
Years later, he described the experiment:
When I broke the seal between the red PtF6 gas and the colorless xenon gas, there was an immediate interaction, causing an orange-yellow solid to precipitate. At once I tried to find someone with whom to share the exciting finding, but it appeared that everyone had left for dinner!”20
There was little fanfare at the time for his accomplishment. He announced his discovery in one of the briefest scientific papers of its kind, coming in under 250 words.21 22
But it was enough for other chemists to take notice and quickly verify his findings, and it was hailed as a landmark in chemistry’s history. It opened the doors to an entirely new specialty in the field, and today there are more than one hundred different compounds of noble gases.23
He never won a Nobel Prize for this achievement, even though it’s widely held that he deserved it. He earned other accolades, though. The site of the experiment has been designated an International Historic Chemical Landmark, and Chemical & Engineering News named it “one of the ten most beautiful experiments in the history of chemistry.”24 25
Perhaps the most impressive honor he received came from the University of California, Berkeley, where he spent decades as a professor. When Bartlett died in 2008, the university published a notice remembering his life and work. Within its text was a limerick:
There was a young man of Vancouver
Who devised a clever maneuver.
He showed that a gas he was keen on
Could even react with xenon.
Thus he greatly enhanced his whole oeuvre.26
Xenon is one of the few elements that would make your collection stand out more if you managed to secure one of those compounds. But even in its elemental form, there are plenty of ways to secure a satisfactory sample of element 54.
You might find it in a nearby hospital, because xenon makes a surprisingly good anesthetic. Breathing a mixture of 80% xenon, 20% oxygen produces a potent painkilling effect. It brings about unconsciousness swiftly, and patients cheerfully rouse from their sleep only two or three minutes after administration ends. It’s also healthier for the brain, heart, and other organs than nearly all other anesthetics.27 28 29 30
The main drawback with this wonder-drug is that it’s pretty expensive, owing to its rarity. But that can be solved: Since xenon is an inert gas, what you exhale is the same as what you inhaled. It’s not like oxygen, which the lungs convert to carbon dioxide. So with a little clever engineering, the exhaled xenon can be recaptured and pumped back into the breathing circuit.31
As we heard at the top of the show, it’s not only patients going under the knife who huff xenon. In addition to the hormonal effects, breathing a xenon-based air mixture can mimic the effects of training at altitude. That is, by breathing an air mixture with less oxygen than normal, the body learns how use the oxygen it gets more efficiently. This increased efficiency provides a performance boost when the athlete returns to a more typical, oxygen-rich environment.
The acceptability of this practice is a contested issue in the world of sport. But there is another, less controversial way that xenon could propel you to great speeds: As part of the engine that drives a spacecraft.
It’s called an ion engine, and it works by positively charging a cloud of atoms, then using an electrical field to fire them into space. The spacecraft is thrust with equal force in the opposite direction.
An atom of xenon is massive. Literally. As in, it carries a lot of mass. It’s nearly ten times heavier than the nitrogen that constitutes most of the air you’re currently breathing. Ejecting heavy atoms provides thrust far more efficiently than lighter atoms would, so xenon makes a great propellant.
Here’s a bit of movie trivia: The TIE fighters from Star Wars have that name not because they look like bow ties, but because they have Twin Ion Engines.
Unfortunately, not everything in Star Wars is scientifically accurate. While the Empire’s TIE fighters dart around space with the agility of a caffeinated squirrel, a spacecraft driven by real ion engines can only change course very, very slowly. The thrust provided is about the same as the force it takes to press a key on a computer keyboard.32
That might sound worthless for pushing spaceships around, but it applies this force constantly, and for a very long time. Given enough time, it can achieve speeds ten times faster than the Space Shuttle was capable of.
But there’s no way it could shake an X-Wing on its tail. Sorry to break your suspension of disbelief. If it’s any consolation, ion engines do create a soft blue glow, almost exactly like the engines on a Star Destroyer. So that’s cool!
Cool, but worthless for you, unless you spend a lot of time at NASA’s Jet Propulsion Lab. For everyone else,xenon is used in a surprisingly diverse array of easy-to-find light bulbs.
There are the so-called “neon” lights, of course, which are actually filled with a variety of gases to imbue different colors. Rather than the “blaze of crimson light” provided by true neon, xenon gives off a bright white light with just a tinge of blue.33
That’s valuable for more than just colorful signage. It turns out to be almost exactly the same color as sunlight, so our eyes see it as more neutral than a fluorescent or incandescent bulb.
Photographers are especially interested in that kind of thing. So with its neutral color and intense brightness, xenon makes the perfect medium for a camera’s flash. Practically every flash that’s ever burned your retinas was running on xenon.
Except for the smartphone cameras, that is. Those are LEDs, which are cheap and energy-efficient, but not actually very good at lighting a scene. For a quality image, you can’t beat xenon.
On this matter, the projectionist can agree with the photographer. IMAX theaters show their movies on screens far larger than the standard multiplex, so only the best and brightest will do.
An IMAX projector employs two 15,000-watt liquid-cooled xenon lamps. Two feet long and ten pounds each, their quartz glass bulbs contain that gas under thirty atmospheres of pressure. That all adds up to a device that demands a great deal of caution when handling.
It takes at least two people to change an IMAX bulb — no joke — and they both need to wear full-body protective gear, because dropping the bulb can cause it to shatter with the explosive force of a grenade.
It would make an impressive showpiece in your gallery of the elements, but considering how many hazardous samples we’ve already collected, and how many more are yet to come, you might not want to imperil yourself any more than necessary.
Thanks for listening to The Episodic Table of Elements. Music is by Kai Engel. To learn how to collect multiple noble gases in one bougie beverage, visit episodic table dot com slash X e.
Next time, we’ll get really blue with caesium.
Until then, this is T. R. Appleton, reminding you that, at the end of the day, when the lot’s all full, and everybody’s fighting to get outta here, we’ll be the first ones out!
Sources
- Slate, Did The Russians Have A Secret Performance-Enhancing Weapon In Sochi? Josh Voorhees, February 26, 2014.
- Public Radio International, Some Endurance Athletes May Be Huffing Xenon Gas To Gain An Edge. Bradley Campbell, December 5, 2014.
- The Irish Times, Russian Athletes Admit Xenon Doping At Winter Olympics. Johnny Watterson, September 10, 2014.
- From Nobel Lectures, Chemistry 1901-1921, Elsevier Publishing Company, Amsterdam, 1966
- Chemicool, Xenon Element Facts. October 18, 2012.
- Some Ninetheenth Century British Scientists: The Commonwealth And International Library: Science And Society, p. 248-249. R. Harre, 2014.
- The Periodic Table: A Field Guide To The Elements, this page. Paul Parsons and Gail Dixon, 2013.
- Skeptoid Blog, How Do You Make An Atomically Sharp Needle? Brian Dunning, September 3, 2014.
- The New Quantum Universe, p. 82. Anthony J. G. Hey and Patrick Walters, 2003.
- Eigler, D. M., & Schweizer, E. K. (1990). Positioning single atoms with a scanning tunnelling microscope. Nature, 344(6266), 524–526. doi:10.1038/344524a0
- The New York Times, 2 Researchers Spell ‘I.B.M.,’ Atom By Atom. Malcolm W. Browne, April 5, 1990.
- IBM, IBM Spelled With 35 Xenon Atoms. September 28, 2009.
- Wired, 20 Years Of Moving Atoms, One By One. Priya Ganapati, September 30, 2009.
- CNET, IBM’s 35 Atoms And The Rise Of Nanotech. Stephen Shankland, September 28, 2009.
- The New York Times, Gas Xenon Is Converted To A Metallic Form By Scientists At Cornell. Malcolm W. Browne, November 16, 1978.
- Bogomolov, V. N.. Metallic Xenon. Conductivity or Superconductivity. (1999).
- LibreTexts Chemistry, 2.11: Metals, Nonmetals, And Metalloids. Mike Baber and Binod Shrestha, June 5, 2019.
- Thanks to ScienceAnalogies.com for presenting this analogy when I couldn’t think of anything appropriate to illustrate atomic radius and ionization energy.
- Chemistry World, Xenon Hexafluoroplatinate. Hayley Bennett, September 29, 2015.
- Fluorine Chemistry At The Millennium: Fascinated By Fluorine, p. 38. Edited by R. E. Banks, 2000.
- Hargittai, I. Neil Bartlett and the first noble-gas compound. Struct Chem 20, 953 (2009). https://doi.org/10.1007/s11224-009-9526-9
- Proceedings Of The Chemical Society, Xenon Hexafluoroplatinate(v) Xe+[PtF6]-. Neil Bartlett, June 1962.
- Sampson, M. (2006) Beginnings. Neil Bartlett and the Reactive Noble Gases. American Chemical Society
- American Chemical Society National Historic Chemical Landmarks. Neil Bartlett and the Reactive Noble Gases.
- Nature, Neil Bartlett (1932-2008). Karl O. Christe, 2008.
- In Memoriam: Neil Bartlett. William L. Jolly, 2008.
- Stoppe, Christian & Rimek, Achim & Rossaint, Rolf & Rex, Steffen & Stevanovic, Ana & Schälte, Gereon & Fahlenkamp, Astrid & Czaplik, Michael & Brülls, Christian & Daviet, Christian & Coburn, Mark. (2013). Xenon consumption during general surgery: a retrospective observational study. Medical gas research. 3. 12. 10.1186/2045-9912-3-12.
- Xenon: no stranger to anaesthesia R. D. Sanders, N. P. Franks, M. Maze. BJA: British Journal of Anaesthesia, Volume 91, Issue 5, November 2003, Pages 709–717, https://doi.org/10.1093/bja/aeg232 Get it? No “STRANGER” to anaesthesia? Eh? EH????
- More on xenon’s mechanism of action: Xenon: elemental anaesthesia in clinical practice. Robert D. Sanders, Daqing Ma, Mervyn Maze British Medical Bulletin, Volume 71, Issue 1, 2005, Pages 115–135, https://doi.org/10.1093/bmb/ldh034
- Franks, N. P., Dickinson, R., de Sousa, S. L. M., Hall, A. C., & Lieb, W. R. (1998). How does xenon produce anaesthesia? Nature, 396(6709), 324–324. doi:10.1038/24525
- The Association Of Anaesthetists Of Great Britain And Ireland, Xenon Anaesthesia For All, Or Only A Select Few? A. E. Neice and M. H. Zornow, 2016.
- Glenn Research Center, Ion Propulsion: Farther, Faster, Cheaper. December 7, 2004.
- OpenLearn, Noble Gases. July 17, 2007.
Arsenic imlplicated in illness of Clair booth luce
Ambassador to Rome
When will get to the superheavy elements?
Assuming I don’t get knocked off-track, we should hit the transactinide elements sometime around the end of 2021 — and that’ll be the home stretch for the final fifteen elements of the periodic table!
Are all period 7 elements are metals?
It’s possible! The short answer is that everything beyond hassium is too short-lived for us to know its physical qualities like that.
That’s the last noble gas ever?
There’s radon, which is radioactive but also a noble gas, and finally oganesson. Oganesson could be a noble gas, but it’s too volatile for us to study it very well. It’s also the largest element we’ve discovered yet.
In this episode you can hear the best metaphoric examplation of ionisation energy I’ve ever heard. I look forward for a parking lot where the spots are labeled like 1S, 2S,2P and so on 😉
By the time you get to 4s, the parking lot is basically a hyperdimensional Mobius strip.