Podcast: Play in new window | Download
We all know that radioactive rocks glow in the dark, except they actually don’t, except for when they actually do.
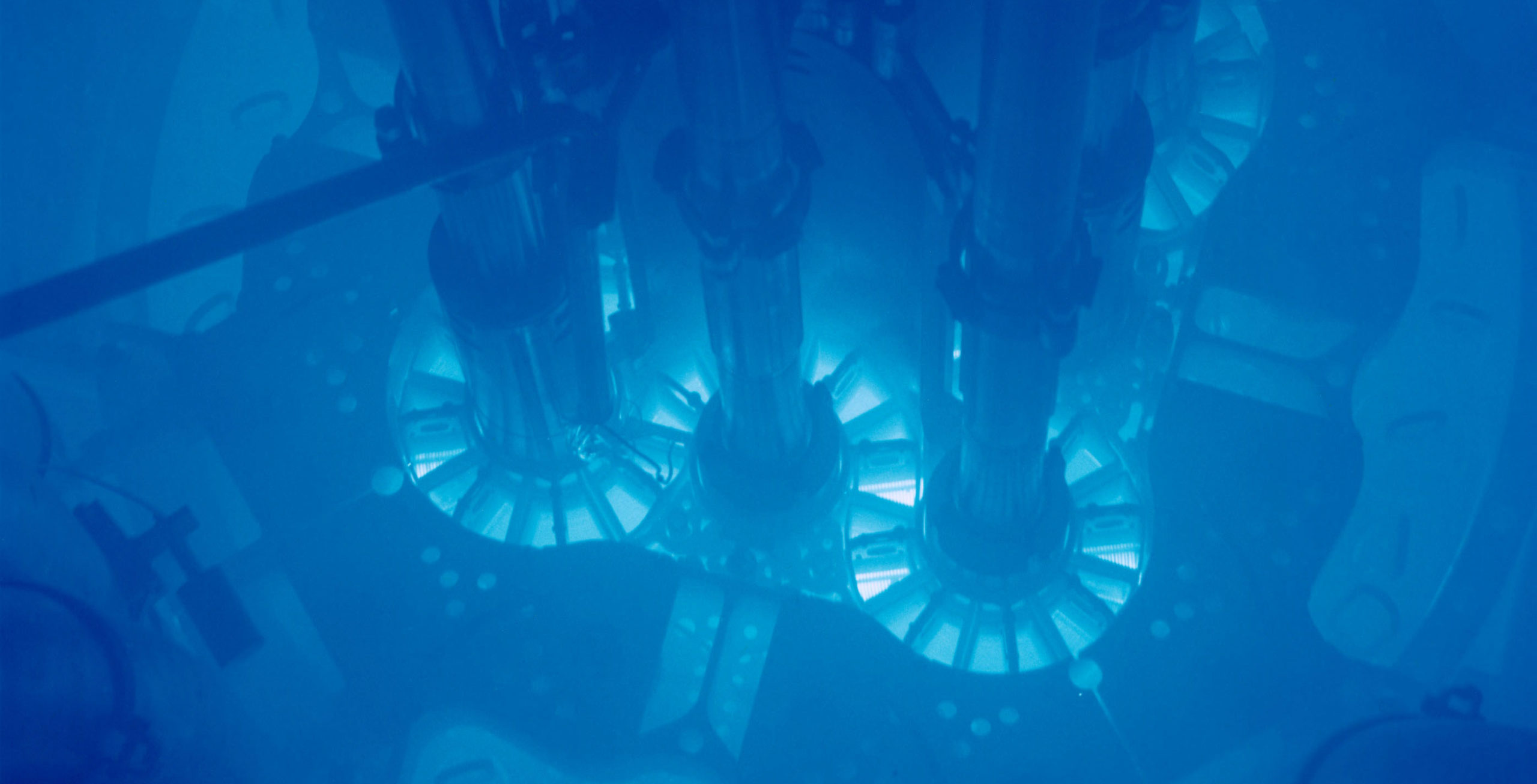
Featured above: A real photograph of Cherenkov radiation, although this particular example is not produced by today’s element. See below for the real deal.
Show Notes
 The usual — show notes and sources are on their way!
The usual — show notes and sources are on their way!
In the meantime, the photo at the right shows a genuine sample of actinium with its characteristic blue glow, thanks to Oak Ridge National Labs. (Click for big.) They’re among the very few who could produce such a sample!
Episode Script
For a long time after its discovery, radium was practically synonymous with the very idea of radioactivity. The catchy name helped, along with its presence in so very many consumer products. You could say that it cast a very long shadow, culturally speaking.
That shadow probably has a bit of a green tinge to it, in many people’s minds. A viridian glow has become visual shorthand connoting radioactivity. Those very popular glow-in-the-dark watches we discussed last time probably had something to do with it.2
It’s an effective way to communicate the idea, but it’s not especially accurate. Radioactive elements don’t naturally glow a bright green color. Even those radioluminescent watch dials required a layer of zinc sulfide in order to pull off that trick. So whatever Homer Simpson is handling down at the Springfield power plant, it probably makes for lousy nuclear fuel.
The one element that actually does follow cartoon logic is actinium-227. It naturally emits a ton of beta radiation — enough to give the surrounding air a faint blue glow.3 4 5
Honestly, it doesn’t really compare to the bright toxic green we all associate with radioactivity. That’s okay, though — actinium is totally cool doing its own thing. As we’ll see, today’s element isn’t afraid to stand apart.
You’re listening to The Episodic Table Of Elements, and I’m T. R. Appleton. Each episode, we take a look at the fascinating true stories behind one element on the periodic table.
Today, we’re
We should pause for a moment before blowing past that characteristic blue glow, because it really is a fascinating phenomenon in its own right.
It happens when a charged particle exceeds the speed of light. You may remember that beta particles are simply electrons (or their antimatter equivalent, positrons). Actinium-227 emits beta particles with so much energy that it lights up the surrounding environment.
“But wait,” I hear you cry, “nothing can exceed the speed of light!”
An astute observation, listener. And it’s true: As far as we can tell, it’s impossible for matter to travel faster than the speed of light …in a vacuum. When light travels through a material, such as glass, or water, or air, it slows down — sometimes by a lot. But the cosmic speed limit remains constant. So while photons slow down like a nervous driver on the highway, other particles are free to speed on by.
Actinium isn’t breaking the speed limit, it’s just going faster than light can go in air (or water, or whatever). To simplify a bit, the blue glow is like the sonic boom that happens when an airplane breaks the speed of sound.
The phenomenon is called Cherenkov radiation, after one of the first people to notice and study the strange blue light. But he wasn’t the first person to find actinium. That was… somebody else.
Pitchblende is a material that’s come up in several recent episodes, since the Curies studied it so extensively. That’s where they first found polonium and radium. First and foremost, though, it’s a uranium ore. There’s even a little technetium in there sometimes, and many other elements, too.
The “blende” part of its name sounds pretty fitting, really, since it quite literally is such a mixture of different atoms. That’s not what it means, though. “Pitchblende” comes from German, with “pitch” meaning “black,” and “blende” meaning “to deceive.”
It earned this underhanded reputation from the miners who first encountered it. That black color made the mineral look like lead — which is what they were looking for — but there was no lead to be had when they processed the ore.
The name was also a bit of a pun, because the similar-sounding word “pech” means “bad luck.”6 Personally, I find this rather amusing, like it was named by Yosemite Sam: “Why, that no-good bad-luck lyin’ little sassafrassin so-and-so!”
For turn-of-the-century chemists, though, pitchblende provided far better fortunes. (At least, if you ignore all the cancer.) Even after finding two new elements in the mineral, there was still plenty to be discovered in the Curies’ castoffs.
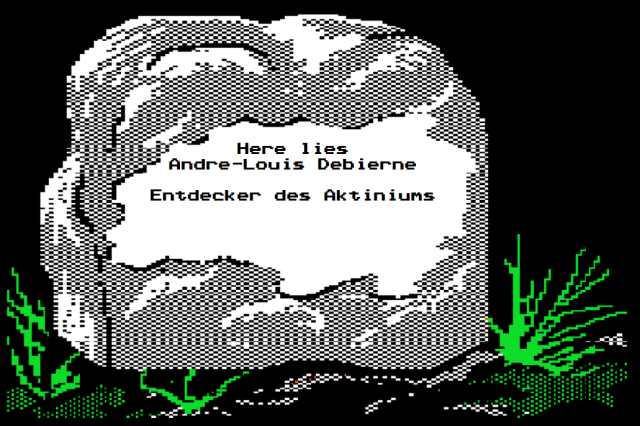
Their friend André-Louis Debierne suspected that might be the case. (You might remember him from two episodes back — he was one of the scientists who had the privilege of calling Marguerite Perey his lab assistant.) He started collecting residue left on the lab equipment the Curies used for their experiments. After ample investigation, Debierne believed he had discovered a new element. He named it “actinium” from the Greek “aktis,” which means “sunbeam” — an allusion to its high radioactivity.7
Meanwhile, in Germany, Friedrich Oskar Giesel was independently conducting similar work. A short time after Debierne’s announcement, Giesel made a similar announcement, calling his discovery “emanium” for the way it “emanated” radiation. You could say that Giesel and Debierne were on a similar wavelength.
I’m sorry, I’m sorry.
Some other scientists got together and compared Giesel’s new element to Debierne’s new element. Seeing that they had similar half-lives, they concluded that both men had discovered the same element. Since Debierne’s finding came first, his name stuck, and “emanium” faded away.
It’s not quite so simple, though. Debierne had observed similarities to titanium and thorium, while Giesel had noted chemical behavior similar to lanthanum. Those are all in roughly the same neighborhood, but not really the same.
Knowing what we know after a hundred years of study, it actually sounds like Debierne might’ve found not actinium, but protactinium — element 91, which wouldn’t be identified for several more years. Giesel’s results sound more like the element we know as actinium. By all rights, we should know the element as emanium; alas, there is as much justice in the history of chemistry as in the history of everything else.
Writer Jean-Pierre Adloff suggests we take a less adversarial perspective. He points out that Debierne was not exactly braggadocious in his original claims, and these guys were discovering an entire new field of science, and anyways, no one can really prove that Debierne’s sample didn’t contain actinium.8
It probably provided little consolation to Giesel himself, but there is one place where he is unequivocally recognized as “The Discoverer of Actinium”: His tombstone.
There have been other controversies surrounding element 89, but they’re all pretty low-stakes. No duels fought in the streets of Paris over actinium.
Some of these scandalettes will sound rather familiar. This series of elements, like the lanthanide series, takes its name from the one at the front of the line. Elements 89 through 103 are called the actinides.
Well, really, the IUPAC would prefer that you call them the actinoids, rather than actinides. For one thing, the -ide suffix usually indicates that something is an ion, like fluoride or sulfide. And to get even more pedantic, from its Latin roots, “actinide” would mean “sons of actinium,” whereas “actinoid” would mean “similar to actinium.”
They also insist that actinium can’t be an actinoid for the same reason lanthanum can’t be a lanthanoid: It can’t be like actinium if it is actinium.
But old habits die hard, so the IUPAC concede that it is acceptable to refer to actinium and all its ilk as actinides. I was always told that you should “avoid the Noid,” so this program will conform to popular usage.
While it might seem obvious that the elements immediately following radium would fall in place under those elements immediately following barium, this wasn’t always clear. In the 1940s, there were still a lot of vacancies in the periodic table, and even those elements that had been found weren’t entirely understood. Thorium, protactinium, and uranium were commonly placed in the slots where rutherfordium, dubnium, and seaborgium live today.
Glenn T. Seaborg was not the first person to suspect their proper placement, but he was the first to provide robust evidence suggesting that the telements surrounding uranium might belong in another series similar to and directly below the lanthanides.9 10 12
That’s all I’ll say about that today, but you’ll want to remember that name — Glenn T. Seaborg — because he’s going to pop up a lot as we traverse the actinides. It’s not a coincidence that one of those elements is named “seaborgium.”
Back to today’s element, the one that kicks off the series, there aren’t many avenues open to the element collector. Most of the time, actinides can really only be found in one of two places: Nuclear power plants, and nuclear weapons.
But let us not be dissuaded! There is one industry that employs esoteric isotopes that’s a little more accessible to the average civilian: Medicine.
Actinium sees some oncological use. Its powerful radioactivity can annihilate cancer cells, and it can be precisely delivered to those cancer cells thanks to the power of monoclonal antibodies.
You may have heard about those recently — monoclonal antibodies are having a bit of a moment, thanks to their ability to fight COVID-19. But even breathless news reports of their efficacy often don’t explain what monoclonal antibodies actually are.
Thankfully, they’re not terribly difficult to understand. The antibodies in question are white blood cells. Scientists will specially seek out and cultivate white blood cells that go after highly specific targets, like the SARS-CoV-2 coronavirus, or blood cancer cells. When a suitable antibody is found, technicians get to work making clones. All monoclonal antibodies of a particular type are identical, and can be traced back to a single ancestor.
The next step is devising the actual medicine to be delivered. In this case, that’s a special compound of actinium that easily attaches to the antibody, hitching a ride straight to the cancer cells. Actinium’s radiation radiation is strong, but very short-range, so it destroys the cancer cell while keeping collateral damage to a minimum.
Unfortunately, doctors tend to be pretty possessive about that kind of material, so you probably can’t go to the nearest hospital and expect to waltz on out with an armful of actinium-laced antibodies. And there really aren’t any other applications for the element that are accessible to the average citizen.
But perhaps all this talk of pitchblende has you feeling a little warmly toward that mineral. These days it tends to go by the name “uraninite,” and while it’s not the most popular rock on the block, it’s not entirely out of reach.
Actinium is scarce, though. If you got your hands on one ton of pitchblende, only 0.15mg of it would consist of element 89. Even then, I’m afraid you’d need to gather an awful lot more before you could illuminate your element collection by the pale blue light of actinium 13
Thanks for listening to The Episodic Table of Elements. Music is by Kai Engel. To see that azure glow for yourself, visit episodic table dot com slash A c.
Next time, we’ll prove ourselves worthy of thorium.
Until then, this is T. R. Appleton, reminding you that a Geiger counter might come in handy as we plunge into the actinides, and those are actually pretty easy to find.
Sources
- Mental Floss, Where Did The Myth That Radiation Glows Green Come From? C. Stuart Hardwick, April 25, 2018.
- Chemical & Engineering News, Actinium. Greg Wall, Sigma-Aldrich, 2003.
- AzChemistry.com, Why Does Actinium Glow In The Dark? Discovery – Element – Properties. June 6, 2018.
- Gizmodo, This Element Is So Radioactive It Actually Glows. Esther Inglis-Arkell, May 26, 2015.
- Oak Ridge Associated Universities Museum Of Radiation And Radioactivity, Jáchymov: Cradle Of The Atomic Age.
- Isis Volume 62, Number 3, The Discovery Of Actinium. H. W. Kirby.
- Radiochim. Acta 88 123-127, The Centenary Of A Controversial Discovery: Actinium. J. P. Adloff, January 3, 2000.
- Lawrence Berkeley National Laboratory, Origin Of The Actinide Concept. G. T. Seaborg, 1994.
- The Nobel Prize, Glenn T. Seaborg Biographical.
- 11Journal Of The Franklin Institute Devoted To Science And The Mechanic Arts, Recent Research On The Transuranium Elements. Glenn T. Seaborg, December 1963.
- https://www.chemicool.com/elements/actinium.html
Super show! Thanks, TRA.
Thank you for listening! 🙂
Listened through every episode over the past few days and had a lot of fun with it! I learned a lot of history especially.
One mistake though: on the episode archive page, platinum links to the iridium episode. Might want to fix that.
Thank you very much for catching that! Should be all fixed now. I’m glad to hear you’re enjoying the show! 🙂
Maybe it’s recency bias, but lead was one of my favorite episodes. I don’t think humanity has had a relationship quite as tumultuous and complicated with any other element.
It’s definitely a remarkably sordid history, isn’t it! That’s saying something, too, considering how many grim tales are littered about the periodic table.
I’m so glad to hear you enjoyed the episode!
Hi T R,
I’ve been listening to your ramblings for years, pretty well from the get go I guess. This is a long overdue mail just to say “Thanks” and “well done”.
I trained as a scientist and have spent nearly 40 years visualising and explaining technical, medical, engineering developments for commercial clients. I know how challenging this can be in terms of putting information across whilst keeping the interest of the audience. You have developed your own style, often slipping so far off piste that I have to remember what you’re on about, but I say that with some affection as many of your listeners probably appreciate it, as I do.
I’ve recently been through a very dark period with my teenage daughter and acutely ill partner. Listening again to your entire back catalogue has really been a help and has kept me almost sane. Bless you. One rarely knows how one’s life touches others.
Love long and prosper. Maybe take some breadcrumbs along in case you really lose your way one episide… 😁
Long John
I enjoyed your comments, John, and I appreciate that it was worthwhile during this anxious time for you to express gratitude.
I hope your daughter and partner will be okay, or at least as well as their circumstances permit. Good wishes to you, some peaceful moments, whatever will keep you steady and healthy.
And how grateful I am to have listeners like you — thank you, Leynia!
John, I can’t thank you enough for taking the time to write such a kind and thoughtful note. It is truly high praise to hear someone with your kind of expertise speaking well of the podcast — I greatly appreciate it!
And I’m rather humbled to hear that I could provide any kind of diversion during such a difficult time for you. I really would have never guessed — I’m very grateful to you for sharing, and also, I hope all the best for you, your daughter, and your partner during such a difficult time. I hope easier days lie ahead for you.
I’m sure I’ll take the long and rambling way to get wherever it is I’m going. I’m very glad to have you along for the ride!
Peace and long life!
Your podcast is amazing. My son (7) has now listened to all the available episodes with my wife and I. As a side effect, he appears to have memorized large chunks of the periodic table (atomic number and symbol). BTW, I love the tangential nature of a lot of the stories.
This is amazing to read — I can’t tell you what a smile that puts on my face! Thanks to all three of you for listening, and I hope you continue to enjoy when new episodes start to air again!
Will there be an episode 90 (and so on)?
Yes, there will! I’ve been quite preoccupied recently (see here) but I’ve also been hammering away on the script for Thorium. No promises as to when exactly it’ll air, but it’s coming.
Your spirit of humor is extraordinary, right in character with the subtle humor of your productions. Thanks for sharing your chemistry vials. Wishing you smile-making scans and looking forward to your treating us to Thorium.
Good eye, sniper 😉
Sounds good! Was starting to get worried that you gave up on this podcast. Really enjoy these.
I just starting listening to this podcast. It’s very well done, entertaining and educational. Thank you, T.R. Appleton!
Since for the element collector Actinium would be a challenge, can I recommend that a USA Homer Simpson stamp may interest the younger collector in that unique “Blue Glow” of Actinium. Perhaps
more apt the Soviet Union issued stamps to each of the 1958 Nobel Prize Winners in Physics, P. Cherenkov, I. Frank & I. Tamm upon their discovery & interpretation of the Cherenkov effect.
T. R. my congratulations on an excellent episode on a “tough” element.