Podcast: Play in new window | Download
In this episode, learn how Dmitri Mendeleev overcame a difficult childhood to become known as the Father of the Periodic Table.
Show Notes
It may be helpful to have a periodic table to reference while listening to this show. In my opinion, the most useful one on the internet is freely available at PTable.com.
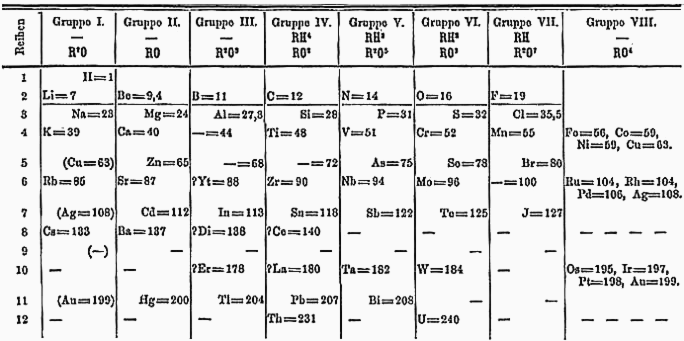
I also promised to include a few examples of non-traditional periodic tables. Click any of the below to view at a larger size:



One of Mendeleev’s original tables is at the top of this page, and hundreds more are available at meta-synthesis.com.
If you have any questions, thoughts, or anything you’d like to see in future episodes, leave a comment below!
Video
To see more episodes in video form, subscribe to The Episodic Table Of Elements on YouTube.
Episode Script
Dmitri Mendeleev might have received the most devastating college rejection letter of all time.
At 16 years old, he had traveled over a thousand miles across the Siberian wilderness to apply to the University of Moscow. He was unquestionably bright, and clearly dedicated, but there was one problem: In 1849, Moscow was a politically turbulent place, and the university wasn’t comfortable admitting someone from so far away — no matter how smart he was. Mendeleev was denied admission.
But he wasted no time lamenting this turn of events. Instead, he traveled 400 miles more, to try his luck at schools in St. Petersburg.
His commitment was rewarded when the Main Pedagogical Institute of St. Petersburg accepted Mendeleev as a student — and gave him a full scholarship, too.
These traits — his diligence, love of education, and sheer force of will — would be instrumental in making Dmitri Mendeleev not just a historic chemist, but the Father of the Periodic Table of Elements.
You’re listening to the Episodic Table of Elements, and I’m T. R. Appleton. Each week, we’ll take a look at the fascinating stories behind one element on the Periodic Table.
But before we start, let’s take a look at the table itself, and how it came to be.
Along with a world map, the periodic table of the elements has adorned the walls of just about every classroom in the past hundred years. And those two make a pretty good pair: While a map can show every where in the world, the periodic table shows you every thing.
That’s not an exaggeration. Everything you have ever seen, from the tip of your nose to the most distant star in the sky, is made up of some combination of these 118 elements on the periodic table. Nothing more, and nothing less. Everything in our lives is built from this modest collection of ingredients.
The periodic table of elements is our best attempt at cataloging these ingredients. Atoms are placed in rows, left-to-right, in order of ascending atomic number. These rows are the periods that give the table its name. For example, hydrogen and helium make up the first period, lithium through neon make the second period, and so on.
The columns, also called “groups,” are the clever part. Elements in any given group behave in really similar ways. For instance: The elements in the rightmost group, from helium down, are all gases without color, odor, or taste, and they barely interact with anything.
Arranging the elements by similarity might seem obvious now, but in the earliest days of chemistry, it was anything but. And arranging the elements in this particular way was exactly what solidified Mendeleev’s name in the history books.
Now, it’s worth mentioning that Mendeleev was neither the only person nor the first person to notice that there seemed to be some sort of underlying pattern to the elements. At least half a dozen other scientists had hit upon the idea before Mendeleev, but being met with ineptitude, or skepticism, or even outrage, they became victims of bureaucracy, unrecognized for their contributions until several years later, if at all.
The crazy thing is, this isn’t even very uncommon throughout history. The jet engine, and the microchip, and the television were all unknowingly invented by at least two separate people independently, often within weeks of each other. It turns out that when a lot of people are reading the latest research at the same time, at least a couple of them are going to stumble across the same answers.123
In fact, this is such a common phenomenon that it has its own name: it’s called Multiple Discovery, and this tends to create a much less dramatic, but more accurate, picture than the one painted by more popular “Great Heroes of History” narratives.
Our love of portraying progress as something accomplished by reckless loners on the brink of madness is simply not true. And in light of that, it would be irresponsible not to mention some of the other minds who were really only slightly less fortunate.
Minds like John Newlands, whose presentation of the elements as following a “Law of Octaves” was a bit too poetic for the Chemical Society of London. They refused to publish his work.4
Alexandre-Émile Béguyer de Chancourtois was — uh, a mouthful, especially for a non-French speaker like myself. But more importantly, he was a geologist. He presented the elements as following a helical pattern that he called “The Telluric Screw,” so called because element 52, tellurium, was at its very center. It was highly accurate, but also pretty complex, and unfortunately, his publisher didn’t quite get it, seeing it only as unnecessary artwork that would be difficult to print.5
And Lothar Meyer was a German chemist who reached many of the same conclusions as Mendeleev, at about the same time. This sparked a heated rivalry between the two that would last for the rest of their lives.6
So, Dmitri Mendeleev: He was far from a lone voice on the frontiers of science, but he certainly did deserve the recognition he achieved. For one thing, he worked like a demon: When he was 27 years old, he realized there was no adequate textbook on the subject of organic chemistry. So he wrote one. A thick one, over five hundred pages long, in two months. And this was no last-minute term paper: This work won him awards, and the book was a huge success in several languages, for decades.7
His mind turned to the arrangement of the elements while he was working on the follow-up to that first smash-hit. At the time, there were 56 separate elements known to scientists, and he was searching for a new way to organize them that made sense. He was so devoted that he stayed up late nights and into early mornings. Ironically, it was only when he finally fell asleep that he figured it out. Later, he would write,
“I saw in a dream, a table, where all the elements fell into place as required. Awakening, I immediately wrote it down on a piece of paper.”8
And to be fair, his arrangement of elements was more comprehensive, and more accurate, than any that had come before it. He did two things differently from his predecessors The first: Rather than ordering the elements strictly by their weight, he primarily grouped them by their behavior, rearranging the elements to fit his theory without much justification. This was highly controversial at the time, but it turned out to be the right call.
Secondly, he used this arrangement to predict the existence of several elements that had yet to be discovered, like gallium, and germanium, and scandium, as they would later be named.
And when those elements were discovered, Mendeleev wouldn’t hesitate to get in a fight with their discoverers. When Paul-Emile Lecoq de Boisbaudran published his findings on gallium, they were in disagreement with Mendeleev’s hypotheses about how the element should behave.9
Now, a good scientist might revise his own work in light of this new information, or at least consider the possibility that maybe he’s wrong. But not Dmitri Mendeleev.
No, he started a public feud. He insisted that Lecoq de Boisbaudran must have made some incorrect measurements.
This seems like a fool’s wager, but to everyone’s astonishment — everyone except Mendeleev, anyway — Lecoq de Boisbaudran soon retracted his earlier findings, and corrected them to more accurate values… values which matched up exactly with Mendeleev’s predictions.10
As an aside, not every French chemist has a name that seems deliberately difficult to pronounce, but they will feature prominently as we explore the periodic table. This is likely thanks to the French Society of Chemistry, one of the oldest chemistry societies in the world.
After more than a century of use, it can seem like the periodic table displays some deep, universal truth, sort of like e = mc^2. “These are the elements,” the table says, “And this is how they relate to each other.”
The truth, however, is a little more nuanced than that. It usually is.
First of all, there are hundreds of different presentations of the elements, each one emphasizing something useful for its particular audience. Some of these presentations are in 3D, or even four dimensions. My favorite is by a man named Theodor Benfey, and has the elements arranged in a spiral that helps emphasize the repetitive nature of the elements’ behavior.
I’ll include some examples in the show notes at episodictable.com, if you’d like to see for yourself.
But we’ll mostly be talking about the International Union of Pure and Applied Chemistry’s standard table — the one you’ve known since childhood. For as neat and orderly as it appears to be, it’s riddled with exceptions and controversy. Groups — again, those are the columns — show stronger trends than periods… except for the lanthanide and actinide rows, where it’s exactly the opposite. And if you’re looking at the metalloids, common properties don’t follow horizontal or vertical trends, but a meandering south-east diagonal line down the table. Element 74 is officially called tungsten, not wolfram, even though its symbol is the letter W. And where should hydrogen go, anyway?
These ambiguities are not flaws in the periodic table, but the opposite. Courting controversy with every block that’s placed, the table reflects the values of the people who drew it and the times they lived in.
In doing so, the Periodic Table is more than just an array of the chemical elements that build our universe. Inadvertently, it also shows off those elements that make us human.
Thanks for listening to the Episodic Table of Elements. Next time, we’ll cover three quarters of all matter in the universe with hydrogen, and learn how you can start collecting fundamental bits of the universe for yourself. To read show notes and transcripts, comment on episodes, and learn more, visit episodictable.com.
This is T. R. Appleton, reminding you to study foreign languages when it doesn’t matter so you can sound like you know what you’re saying when it does.
Sources
- The microchip: history-computer.com (archived)
- The jet engine: Fox News, Coincidence? The 9 Craziest Cases of Simultaneous Invention
- The television: Safari Books Online, The Challenge of Simultaneous Invention
- Information on John Newlands from PeriodicTableDay.org
- De Chancourtois’ Telluric Screw, from dataphys.org
- Information on Lothar Meyer from ChemHeritage.org
- The Robinson Library: Dmitri Mendeleev, Creator of the Periodic Table
- Khan Academy: Dmitri Mendeleev
- Dmitriy Mendeleev: A Short CV, and A Story of Life. Eugene V. Babaev, Moscow State University.
- Slate: Blogging the Periodic Table – Gallium: It Proved That Dmitri Mendeleev, Father of the Periodic Table, Wasn’t A Crackpot. Sam Kean, July 20, 2010
This is a great idea, and a beautifully put together series of programmes.
I’d like to use these ‘episodes’ as background briefing for a programme of events we have to commemorate the 150th anniversary of the publication of the table – a landmark in the development of a “unified theory of everything’ .
We are launching a competition – sorry, folks, but its just for folk who live or work in Cornwall, UK – to convey these elements not with names and letters, but as musical notes, so that we can make a whole new kind of landscape music.
The element (sic) of humour and intrigue in these programmes is just right for the tone of our work!
That sounds like a lot of fun! I’d love to hear more about the project and how I can help you out with it. If you don’t mind, please send me a message at contact@episodictable.com and we can chat over email. Thanks so much for your kind words!
Sending you moon-shine from midnight in Minneapolis.
Why thanks 😊 I know it’s been ages between episodes, but I’m still here and working on them!
T.R. – Do you have a Patreon or similar? I’d love to support your fantastic work financially.
p.s. So glad to see the latest episode and that you’re still plugging away a it!
Why, thank you — for the kind sentiments, and for listening! I’m pretty pleased to be back at it, too.
I would love to have a Patreon someday, but I decided at the outset that this would be a project with no financial component. (That’s why there are no ads; unfortunately, that’s also why an extended hiatus can happen.)
It is very helpful to know that there’s any amount of interest in a Patreon, though. For now, telling someone else about the show or leaving a review on Apple podcasts would be of great help — but really I’m just glad for your listenership!